SOLOJEKT AD®
AD SYRINGE WITH NEEDLE
- Description
- Specifications
- Compliance
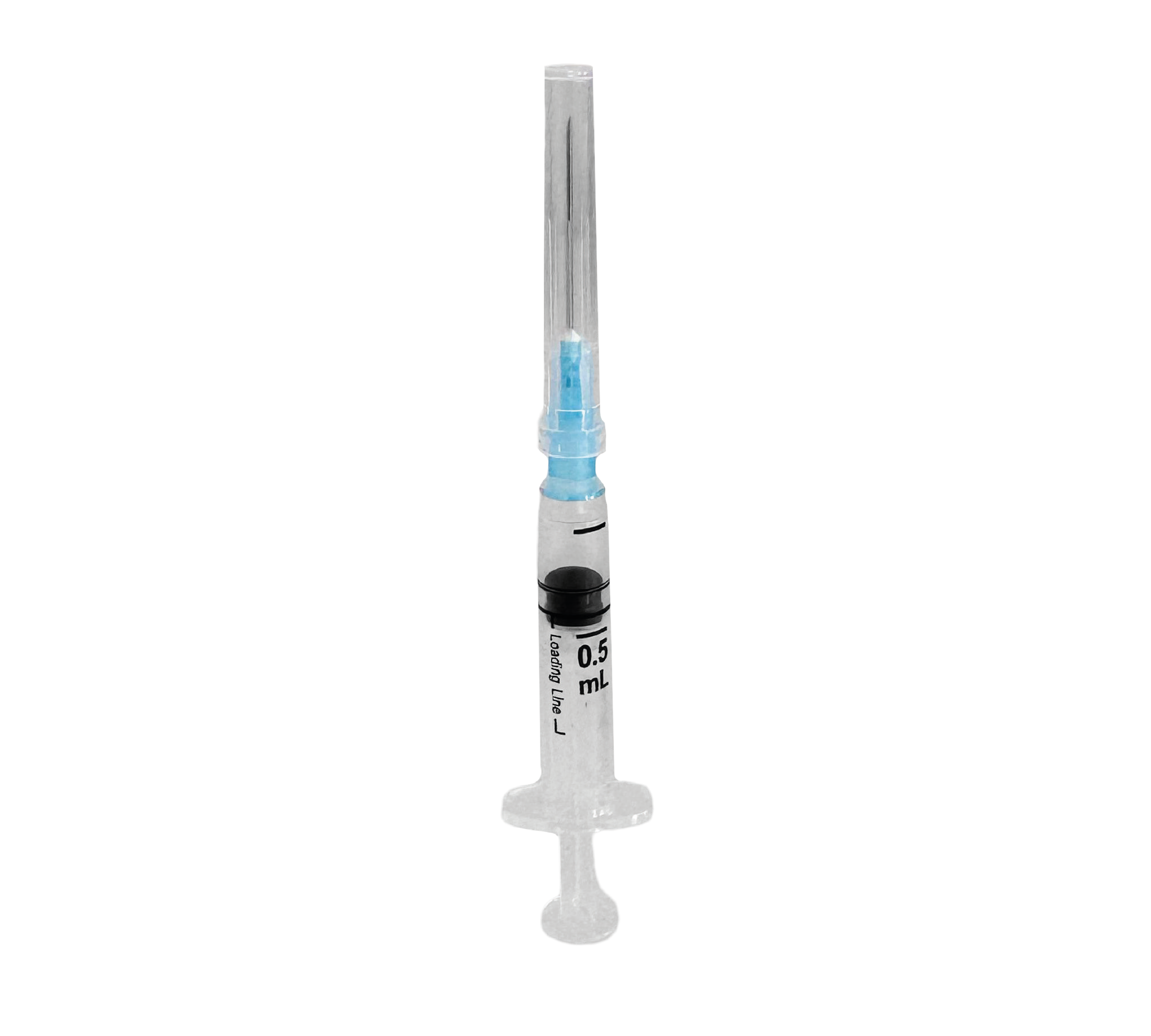
The SOLOJEKT AD® 0.5 ml syringe is a sterile device intended for fixed-dose immunization. It features a transparent barrel and a plunger equipped with an integrated reuse prevention mechanism (RUP) that activates automatically at the start of injection to ensure single-use only
- Component materials:
- Syringe body: Medical-grade polypropylene (PP);
- Piston: Medical-grade polypropylene (PP);
- Piston seal: Thermoplastic elastomer (latex-free);
- Lubricant: Silicone (less than 0.25 µg/mm²);
- Markings: Permanent ink;
- Latex-, PVC- and DEHP-free.
- Component materials for uncrimped needles:
- Needle: Stainless steel;
- Needle base: Color-coded polypropylene (PP);
- Needle cap: Polypropylene (PP);
- Glue: Epoxy resin;
- Latex-, PVC- and DEHP-free.
- Device type: Single-use sterile medical device.
- Sterilization: By ethylene oxide.
- Shelf life: 5 years after sterilization.
- Packaging: Individually packaged in blister packs, boxes of 100.
- Storage conditions:
- Store at room temperature, non-condensing;
- Do not expose to moisture or direct sunlight.
- Certification: ISO 7886-1 ; ISO 7886-3 ; ISO 7864 ; ISO 13485 : 2016.
- IMD-PQS Code: E008-125.
- Biocompatibility: In accordance with ISO 10993 standards.
- Ministry of Health registration no.: 14748/2024/9196-2024/DM/DPS/DMP/18.